
1. Abstract
There is considerable interest in finding natural ingredients capable of restoring and maintaining skin health via modulation of the skin microbiome. The skin surface is host to an array of microorganisms including bacteria, viruses, and fungi. One of the most prevalent bacterial species is Cutibacterium acnes, which has been linked to flare-ups of acne vulgaris as a results of excessive growth, stimulation of inflammation, and/or biofilm formation. Although antibiotics are prescribed frequently for treatment of severe acne, clinicians are hesitant to rely upon these drugs and many strains of C. acnes already have evolved antibiotic resistance, forcing scientists to explore alternatives. An ancient Chinese remedy in the form of the plant Epimedium sagitattum (or horny goat weed) was examined for its touted anti-microbial and anti-inflammatory properties. An extract of E. sagitattum was developed and evaluated in vitro for its potential anti-biofilm, anti-inflammatory, and anti-oxidant features. A dilution series of the extract was utilized in a Minimum Biofilm Eradication Concentration (MBEC) assay with biofilms produced by C. acnes. Relative to negative control standards and a formulation with no known associated anti-biofilm activity, E. sagitattum extract delivered a 4-log reduction in C. acnes biofilms. In addition, the dilution series was applied to human keratinocytes that were stimulated with interferon-gamma (IFN) and muramyl dipeptide (MDP) to determine its effect on bacterial activation of multiple inflammatory factors. The findings from this effort were corroborated by complementary biochemical tests, confirming that the extract down-regulated no less than 6 different inflammatory mediators and pro-inflammatory molecules, including: PLA2, MIP-1β, MCP-1, IL-1β, IL-6, IL-8, GM-CSF, and COX-2. The extract also showed significant anti-oxidant effects. Based on these findings, it was concluded that E. sagitattum extract, subsequently named Grandiciin®, reduces biofilms formed by C. acnes. Furthermore, the anti-biofilm activity complements the capacity of Grandiciin® to diminish levels of multiple mediators of skin inflammation. Combining all of these features, Grandiciin® makes an exciting new ingredient for modulating the skin microbiome and promoting balanced skin health when formulating for blemish-prone skin.
2. Results

Figure 1.
Schematic of comedone formation. The presence of the anaerobic bacterium Cutibacterium acnes has been long considered one of the major causative factors in acne vulgaris (AV). Another factor that contributes to pathology in AV is biofilm created by C. acnes in the pilosebaceous gland. Biofilm is a secreted, polysaccharide-based matrix that adheres to tissues and protects embedded bacteria from germicidal exposures and host immune surveillance.
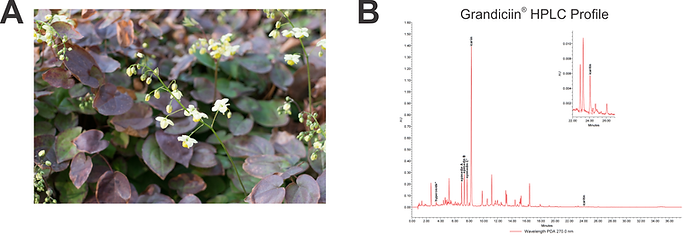
Figure 2.
Botanically-derived ingredient. Grandiciin® was created from a plant rooted in Traditional Chinese Medicine (TCM). A. Epimedium sagittatum is a robust perennial plant that is native to Central China. It is one of several species of this genus that are used extensively in TCM. B. The chromatogram reveals the presence of icariin, epimedin A, epimedin B, epimedin C, hyperoside (galactoside of quercetin) and icaritin (fully de-glycosylated icariin) in Grandiciin®.

Figure 3.
Anti-biofilm assay. A. Schematic of the Minimum Biofilm Eradication Concentration assay. B. The results obtained correspond to a reduction in the Cutibacterium acnes population within biofilm of >120X, >1200X and >2400X when 0.63%, 1.25% and 2.5% Grandiciin® is applied, respectively.

Figure 4.
Anti-inflammatory assays. Grandiciin® inhibited production of six (6) different cytokines in an immunoblot array and were corroborated by ELISAs with an IC50 value below 0.01% in every case. This indicates Grandiciin® has strong anti-inflammatory activity.

Figure 5.
Inhibition of pro-inflammatory compounds. Production of pro-inflammatory prostaglandin E2 (PGE2) requires release of arachidonic acid from membrane lipids through phospholipase 2 (PLA2), followed by conversion by cyclooxygenase-2 (COX-2). In response to C. acnes exposure, increased PLA2 and COX-2 activities are observed. A fluorometric assay measured the effects of varying levels of Grandiciin® on PLA2 and COX-2.

Figure 6.
Anti-oxidant activity. Grandiciin® was tested for its ability to scavenge SO and prevent damaging oxidation reactions. Reactions with a photosensitizing dye, varying concentrations of Grandiciin®, and iodide ion as oxidizable substrate were exposed to visible light. Oxidation of iodide by SO was measured spectrophotometrically. Grandiciin® dose-dependently inhibited SO-mediated oxidation with an EC50 of approximately 0.1%.
3. Conclusions
Grandiciin®:
• contains a variety of compounds with potential benefits to skin health, including icariin; epimedins A, B, and C; icaritin; and hyperoside.
• successfully reduced bacterial biofilms produced by Cutibacterium acnes – the skin microbiome member that is the driving force behind flare-ups of acne vulgaris.
• potently reduced the cellular levels of multiple mediators of skin inflammation including IL-6, IL-8, IL-1β, GM-CSF, MCP-1, and MIP-1β – counteracting the effects of acneic strains of C. acnes.
• also significantly diminished the activity of PLA2 and COX-2 – two critical modulators in the expansion of inflammatory effects – likely resulting in a soothing sensation.
• also showed dose-dependent singlet oxygen (SO) scavenging activity.
• represents an exciting new, bio-active skincare ingredient that is suitably designed to ameliorate blemish-prone skin and fits the model of “clean beauty” desired by modern consumers.
4. References
1) Ma, H., He, X., Yang, Y., Li, M., Hao, D., and Jia, Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharacol (2011) 134: 519-541.
2) Li, C., Li, Q., Mei, Q., and Lu, T. Pharmacological effects of pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci (2015) 126: 57-68.
3) Zhao, F., Tang, Y.Z., and Liu, Z.Q. Protective effect of icariin on DNA against radical-induced oxidative damage. J. Pharm Pharmacol (2007) 59: 1729-1732.
4) Kong, L., Liu, J., Wang, J., Luo, Q., Zhang, H., Liu, B., Xu, F., Pang, Q., Liu, Y., and Dong, J. Icariin inhibits TNF- alpha/IFN-gamma induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int Immunopharmacol (2015) 29: 401-407.
5) Burkhart, C.N., and Burkhart, C.G. Microbiology’s principle of biofilms as a major factor in the pathogenesis of acne vulgaris. Int J Dermatology (2003) 42: 925-927.
6) Coenye, T., Brackman, G., Rigole, P., DeWitte, E., Honraet, K., Rossel, B., and Nelis, H.J. Eradication of Propionibacterium acne biofilms by plant extracts and putative identification of icariin, resveratrol, and salidroside as active compounds. Phytomedicine (2012) 19: 409-412.




